Supervisor
Dr Sarah Robinson
Brief Summary
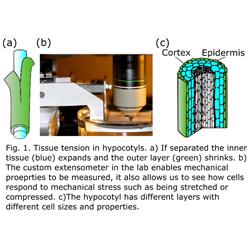
Tissue tension is common in plants. It describes the phenomenon in which the cells within the tissue have a different size or shape than they would if they were growing in isolation. It can result from different growth rates in different parts of the tissue and different mechanical properties of the tissue. Tissue tension is important as it gives structures their strength and can create buckling and 3D shapes. Tissue tension can be seen by cutting the tissues to free the cells from their constraints (Fig. 1a). For example if you peel rhubarb the outside layer will shrink relative to the rest of the tissue. Using the automated confocal micro-extensometer (Fig. 1b) that we developed (Robinson et al., 2017) we applied relative compressive or tensile forces to hypocotyls and saw that the different cell layers responded differently (Robinson and Kuhlemeier 2018)– likely due to the tension already in the tissue. This response resulted in hypocotyls subjected to a small compressive force actually growing faster than control samples or those under tension termed compression-enhanced hypocotyl growth. This response likely helps germinating seedlings sense they are under the soil and push their way to the surface.
Importance of Research
It is also not known how the size and shape of the cells change the mechanical properties of the plant and therefore its ability to withstand abiotic stress such as wind or pushing past an obstacle and how it impacts their growth. By better understanding these interactions we can hope to gain insight that can inform future crop improvement efforts.
Project Summary
In this proposal, we aim to investigate the role of cell size and geometry in generating tissue tension. We will use the Arabidopsis hypocotyl as a model as it has a stereotypical pattern of cell sizes and its growth is well characterised. Tissue tension has often been thought to result from the very large thin-walled cells of the inner layers compared to the smaller thick-walled cells of the outer tissue layers (Fig. 1c). We propose to alter the pattern of cell division specifically in the inner or outer layers to alter the size of the cells in the different layers and see the impact on tissue tension, mechanical stress and growth. The proposed work will also provide insight into the role of cell division in growth generally and the role of the inner tissue layers to overall growth - two topics that have received very little attention.
What will the successful applicant do?
In this project, you will generate synthetic lines in which the usual geometry of the cells is disrupted by changing the frequency of cell division in different tissue layers and changing their growth by modifying the cell wall.
You will characterize the growth of the altered lines to understand how the layer-specific changes to cell division and cell expansion impact the whole tissue. Using a custom-built extensometer in the lab you will characterise the mechanical properties of the altered tissues. Atomic force microscopy will be used to check for local changes to the mechanical properties. You will quantify changes in the pattern of mechanical stress in the tissue and how it responds to external mechanical stress that you will apply with the extensometer.
Training Provided
The applicant will be in an interdisciplinary lab and will learn to use biomechanical tools including atomic force microscopy and the micro-extensometer, synthetic biology and advanced imaging techniques. The applicant will learn how to work in an interdisciplinary environment.
References
- Robinson, S., Huflejt, M., Barbier de Reuille, P., Braybrook, S.A., Schorderet, M., Reinhardt, D., and Kuhlemeier, C. (2017). An Automated Confocal Micro-Extensometer Enables in Vivo Quantification of Mechanical Properties with Cellular Resolution. Plant Cell 29, 2959–2973. https://doi.org/10.1105/tpc.17.00753.
- Robinson, S., and Kuhlemeier, C. (2018). Global compression reorients cortical microtubules in arabidopsis hypocotyl epidermis and promotes growth. Curr. Biol. 28, 1794-1802.e2. https://doi.org/10.1016/j.cub.2018.04.028.
- Kutschera, U., and Niklas, K.J. (2007). The epidermal-growth-control theory of stem elongation: an old and a new perspective. J. Plant Physiol. 164, 1395–1409. https://doi.org/10.1016/j.jplph.2007.08.002.