We investigate how plants measure time by studying a biological 24 h timing device called the circadian clock. We have discovered that the circadian clock improves plant growth, sugars regulate the circadian clock to set a “metabolic dawn”, there is a calcium signalling pathway at the heart of the circadian clock and that circadian clocks are dynamically plastic to allow synchronisation with the external environment of the Earth and to regulate the internal environment of the cell. We perform physiological, genetic, systems and computational studies to understand how the circadian clock is incorporated in to the biology of the cell. We collaborate with colleagues at the National Institute of Agricultural Botany and BASF, Ghent, Belgium to translate our fundamental findings to improve wheat varieties. Projects include:
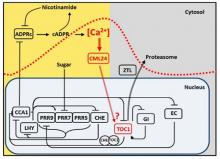
The role of a cADPR- and Ca2+-based signalling pathway that regulates the period of the circadian oscillator
We identified a molecular pathway by which the circadian oscillator generates Ca2+ signals that feedback to regulate the period of the circadian oscillator. We demonstrated that circadian oscillations of cytosolic-free Ca2+ ([Ca2+]cyt) encode photoperiodic information in Arabidopsis (Love et al., 2004 Plant Cell 16, 956 – 966). We subsequently determined that the circadian [Ca2+]cyt signals are downstream of the oscillator, and specifically dependent on CIRCADIAN CLOCK ASSOCIATED 1 (Xu et al., 2007 Plant Cell 19, 3474-3490). We next found that the circadian oscillations of [Ca2+]cyt arise from oscillations of the Ca2+ agonist cyclic ADP ribose (Dodd et al., 2007 Science 318, 1789 -1792). cADPR is an enigmatic signalling molecule that has proven hard to study in any system, but we were able to show that an ADPR cyclase activity capable of generating cADPR is present in plants and is regulated by NO (Abdul-Awal et al., 2016 Plant Physiology 171, 623–631). Our investigation of cADPR signalling suggested that Ca2+ signals might feedback to directly regulate the pace of the circadian oscillator, we formally demonstrated this by describing a new pathway by which cADPR-generated circadian [Ca2+]cyt signals regulate the period of the circadian clock through a calcium binding sensor protein CALMODULIN-LIKE 24 operating in a pathway with the circadian oscillator genes TOC1 and CHE (Marti et al., 2018 Nature Plants 4, 690-698). Taken together we have discovered a Ca based signalling network which is loop within the circadian system. We continue to investigate the molecular basis of this pathway.
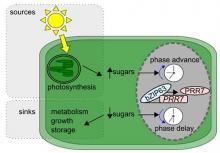
The circadian clock entrains to sugar signals to regulate carbon homeostasis
We discovered and characterised the molecular basis of two new signalling pathways by which sugars regulate the circadian oscillator. Through a combined mathematical modelling and experimental approach we discovered that sugars maintain circadian oscillations in the dark dependent on the action of the circadian oscillator gene GIGANTEA, identifying for the first time a mechanism for metabolic input to the circadian clock (Dalchau et al., 2011 PNAS 108, 5104 - 5109). These effects of sugar in the dark are due to stabilisation of the GIGANTEA protein. Through this study we also found that ethylene also regulates the circadian oscillator, extending the catalogue of regulators of circadian period that we have discovered (Haydon et al., 2017 Plant Physiology 175, 947 - 958). The second metabolic pathway we described entrains the circadian clock to photosynthetically-derived sugar signals (Haydon et al., 2013 Nature 502, 689–692). Our molecular studies have demonstrated that sugar-mediated entrainment of the circadian oscillator occurs through bZIP63 regulation of the circadian clock gene PRR7 with regulation from trehalose 6 phosphate and probably SNRK1 (Frank et al., 2018 Current Biology 28, 2597-2609; Ohara et al., J. Theoretic. Biol 457, 137-151). Through a collaboration with Dr Akiko Satake (Kyushu, University) we have proposed that entrainment of sugars contributes to carbon homeostasis by adjustment of the timing of starch degradation (Webb and Satake 2015 Plant Cell and Physiology 56, 586–593; Seki et al., 2017 Scientific Reports 7, 8305). We are now investigating the role of bZIP transcription factors in setting the pace of the circadian oscillator and the molecular mechanisms by which the circadian oscillator regulates the daily timing of consumption of transient carbon reserves.
Circadian clocks optimise plant performance
We demonstrated that the circadian clock increases assimilation, growth and survival of plants (Dodd et al., 2005 Science 309, 630 – 633). We found that the benefits conferred by the clock are due to the ability to entrain to the oscillating light and dark cycle, rather than the rhythmic behaviour of the oscillator per se (Dodd et al., 2014 New Phytologist 201, 168-179). In collaboration with Professor Malcolm Bennett (University of Nottingham) we demonstrated that the circadian oscillator also confers benefits through the co-ordination of development (Voß et al., 2015 Nature Communications 6). We independently co-discovered that the circadian clock contributes to plant stress responses through the circadian-gating of signalling, in particular modulation of low temperature-induced increases in [Ca2+]cyt (Dodd et al., 2006 Plant J. 48, 962 – 973). Subsequently, we collaborated with Dr Phil Wigge (University of Potsdam, Germany) to show that the circadian clock controls thermo-responsive growth (Box et al., 2015 Current Biology 25, 194 – 199). In collaboration with Professor Jorge Goncalves (University of Luxembourg) we discovered that the circadian clock contributes to seasonal adjustment of gene expression in external co-incidence with cycles of light and dark, which provided global understanding of the circadian control of biological timing (Dalchau et al., 2010 PNAS 107, 13171-13176). This required the development of linear mathematics tools which we used to identify links in the system providing insight in to ELF4 and LUX function (Herrero et al., 2012 Plant Cell 24, 428-443). We are using these insights to understand how the circadian clock might be used to improve wheat varieties through applied and basic research.
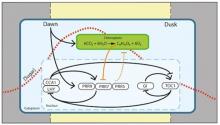
Dynamic adjustment of circadian period contributes to circadian entrainment
In our investigations of the effect of cADPR/Ca2+ on the circadian oscillator we discovered that the metabolite nicotinamide affects circadian period (Dodd et al., 2007 Science 318, 1789 -1792), a finding since confirmed in all the major Kingdoms. Coupled with our findings that sugars and ethylene dynamically adjust circadian period this led to the conclusion that dynamic adjustment of period to biotic and biotic signals might contribute to the functioning of the circadian oscillator. Nicotinamide provided a powerful tool to investigate why circadian period can change in response to metabolites and the role of dynamic adjustment of circadian period in the functioning of the circadian oscillator. By performing an EMS screen, we identified nicotinamide-response mutants, one of which demonstrated that BIG, a protein of unknown function, regulates the dynamic adjustment of the period of the circadian oscillator and this contributes to circadian entrainment (Hearn et al., 2018 Plant Physiology 178, 358-371). In a computational network reconstruction approach, we have identified that nicotinamide regulates the period of the circadian oscillator through affecting the pathways associated with the circadian oscillator genes PRR7, PRR9 and TOC1 (Mombaerts et al. 2019 PLOS Computational Biology). Based on these findings we have introduced the concept of “dynamic plasticity” in which the components of the circadian oscillator are in constant adjustment by biotic and abiotic signals. Dynamic plasticity provides a framework for understanding how the circadian oscillator can integrate with the biology of the cell (Webb et al 2019 Nature Communications, in press). We are investigating the molecular function of the BIG protein and performing computational studies to understand how dynamic plasticity of the circadian oscillator contributes to clock function. We study also other signals that might regulated circadian period, such as magnesium.
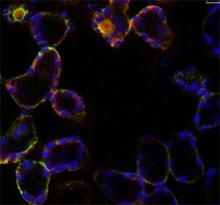
Oscillations of cytosolic-free calcium encode information
We have identified tissue- and stimulus-specific [Ca2+]cyt signals in Arabidopsis by using enhancer-trap technology to target aequorin to different leaf tissues, resolving a long-standing debate as to whether oscillations encode information in leaf cells other than guard cells (Martí et al., 2013 Plant Physiology 163, 625 – 634). This built on earlier studies in which we examined the [Ca2+]cyt signals in plants and made predictions based on mathematical simulations concerning the oscillatory nature of [Ca2+]cyt signals in response to cold and NaCl (Dodd et al., 2006 Plant J.48, 962 – 973; Tracy et al., 2008 PCE 3 1, 1063–1073). Using our cell-targeted aequorin lines, we discovered tissue-specific [Ca2+]cyt signals are evoked by flagellin (Beck et al., 2014 J. Exp. Bot 65: 6487-6498). We have collaborated with Prof Gilliham (University of Adelaide) to demonstrate that correct Ca2+ homeostasis is required for optimal plant growth (Conn et al., Plant Cell 2011 23, 240-257; Hocking et al., 2017 J. Exp Bot. 68, 4171–418). We continue to uncover the basic principles of Ca2+ signalling.