Submitted by Vicki Marshall on Wed, 07/05/2025 - 13:37
The Postdoc Innovation Awards were launched in 2024 to fund innovations by Postdoctoral researchers in the Department of Plant Sciences. Two projects were funded as part of the pilot, and here Unnati Sonawala talks about her project 'Optimizing a high-throughput screen for nematode effector genes in tomato root protoplasts' and what the funding has enabled her to do so far.
Unnati's project
Through the Postdoc Innovation Award, I received support to develop a high-throughput screening method for root-expressed pathogen effectors in tomato root protoplasts.
Overview
Understanding how plants defend themselves against pests and pathogens is essential for developing disease-resistant crops. Plant-parasitic nematodes cause agricultural losses of approximately $80 billion annually worldwide, posing a major threat to global food security. With environmental concerns limiting chemical nematicide use, developing resistant crop varieties has become increasingly important.
While several plant genes conferring resistance (R genes) to different plant-parasitic nematodes have been identified, we often do not know exactly which nematode proteins (effectors) they detect. Without this knowledge, it is difficult to improve existing resistance or engineer new forms of it.
What the award has enabled
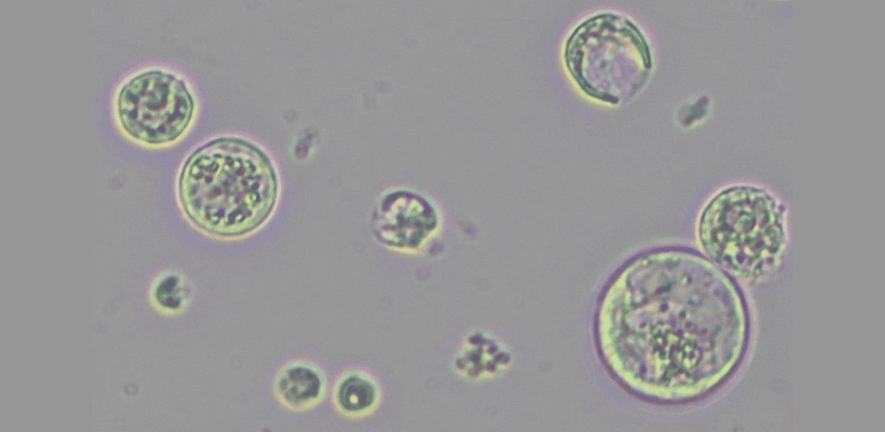
My Postdoc Innovation project focuses on adapting a recently developed, cutting-edge screening method (Arndell et al., 2024) to root cells of tomato (see image), a major crop plant and a key model system for studying nematode infection in the lab. Traditional effector screens use transient expression in Nicotiana benthamiana leaves and require infiltrating each candidate pair of effector and R gene one-by-one, making a large screen slow and labour intensive. By contrast, a high-throughput screen that tests an R gene of interest against a pool of effector candidates directly in root cells - where these effectors are naturally expressed - will uncover these key interactions much more efficiently.
In the first phase of my project, I have successfully isolated viable protoplasts from tomato roots and sweet potato roots - another crop of interest to my primary project. A special thank you to Miguel Santos and Thomas Irving for their help in getting me started on the protoplast isolation protocol.
I am currently working on optimizing their transfection to achieve conditions where enough protoplasts are transfected with both the R gene of interest and a candidate effector. This will be tested using flow cytometry. I will then validate the protocol with RNA-seq and a known R-Avr pair as a positive control, setting the stage for large-scale nematode effector screening in tomato roots.
Importance of the Postdoc Innovation Award
It is often difficult to find funds for testing or optimizing a method within a primary project. These steps come with uncertainty, need for additional reagents, and the cost of validation - all of which apply to this project. The Postdoc Innovation Award has provided me with precisely that opportunity. Furthermore, it has expanded my technical expertise in protoplast and cell biology and will help me add a cutting-edge high-throughput screen to my research toolkit.
Finally, the results from this project will be an important methodological contribution to the field of plant immunity - particularly for studying root-specific plant–pathogen interactions. At last, we can answer the question of whether these R-Avr pairs interact similarly in the native host and tissue!
The Postdoc Innovation Awards will be opening for applications again in September 2025.
Image: Tomato root protoplasts. Photo credit: Unnati Sonawala
